2015
Global Health Innovations
-
The Team
Student Team: Erin Reisfeld, Shruthi Rajan, Dhananjay Sethi, Madeleine Clegg
Clinical Advisors: C. Sridevi, MBBS, DNB, Lalukota Krishna Mohan, MBBS, MRCP, Satadal Saha, MBBS, MS, FRCS, Clifford Weiss, MD, Harikrishna Tandri, MBBS, MD, Naresh Pagidimarry, MS, Peter Johnson, MD, Stuart Russell, MD, Daniel Nyhan, MBBCH, MD, Viachaslau Barodka, MD
Sponsor: Medtronic Team —Val Eisele, Adam Hines, Kesavan Potti, Idara Uko, Yong ChoAbstract
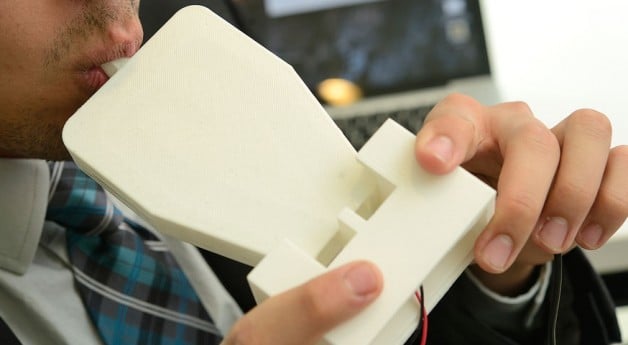
Team Brady is working in collaboration with the Medtronic Foundation to assess and address the barriers to pacing therapies in India. After multiple field studies, the team has observed ineffective management of bradycardic patients in emergency situations. Proper care is stifled by the inaccessibility of temporary pacing, which is a bridging therapy to a permanent pacemaker. In particular, in low-resource settings where imaging technologies and infrastructure do not exist, patients either die before reaching a cardiologist or reach a cardiologist in a life-threatening state.
To address the lack of availability to temporary pacing, Team Brady is developing an assistive device to allow doctors in settings without imaging to safely and confidently temporarily pace a patient. The assistive device is a hybrid needle system that ensures safe venous access by mitigating the potential risks of a blind procedure. The main components of the device are a mechanical needle-syringe system that allows for larger needle integration without compromising the placement on the patient’s neck and a double-aspiration method that allows the physician to confirm access into the jugular vein twice during the procedure. These components as a system were designed to ensure patient safety, encourage physician confidence, and standardize venous access protocols.
-
The Team
Student Team: Wes Bernier, Allie Sibole, Melody Tan, and Jackie Wanjala
Clinical Advisors: Azadeh Farzin, MD, Kusum Thapa, MD, Neena Khadka, MD, Lindsay Litwin, and Kristy PetersonAbstract
In overcrowded neonatal care units where each caregiver is responsible for many babies, there is a serious risk of neonatal distress going unnoticed. Traditional newborn vital sign monitors can alert providers to subtle indications of illness but are too expensive for hospitals in low-resource settings and ineffective in understaffed facilities. We observed this problem firsthand in Nepal and Indonesia and identified a need for caregivers in neonatal units to be able to tell when babies are experiencing vital sign abnormalities in order to know when they are in need of immediate intervention.
As a team of master’s students at the Johns Hopkins Center for Bioengineering Innovation & Design, we are in the process of developing a newborn vital signs monitor for low-resource settings. Our system has three components:
- Wearable vital signs sensors to measure heart and respiratory rate, with potential to add temperature and oxygen saturation monitoring in the future. These sensors are designed to have minimal contact with the baby in order to protect their fragile skin.
- A centralized interface tablet that receives wireless signal from the sensors and displays the vital signs of all the babies in the unit. The centralized interface design would be more affordable than individual patient monitors.
- A paging system to alert caregivers when a baby is in need of immediate attention. From our field observations, we observed that nurses are not always present in the unit. This alert system will enable providers to know if a baby is experiencing distress even if they are not within audible range of the alarm.
We believe that this system will enable earlier detection of neonatal distress, leading to earlier, more effective interventions and alleviating the burden on overworked caregivers.
-
The Team
Student Team: Brian Ma, Qian (Linda) Liu, David Blumenstyk, Krairat (March) Mairin, Trent Langston, and Sriram Chadalavada
Clinical Advisors: Satadal Saha, MD, MBBS, MS FRCS, Soumyadipta Acharya, PhD, Satya Brata Acharya, MD, Aditya Polsani, MS, Michael Parlato, MSEAbstract
Access to health care is a basic human right. Yet in India, more than 700 million rural people have limited access to deficient care with 360 million of them having no access to any form of health care at all, including primary care. Although the government has built a system of free public clinics and hospitals, about 40 percent of them are under-performing. In the absence of quality care facilities, Rural Medical Practitioner with empirically acquired experience and no knowledge base have emerged as a predominant form of care provider for the rural community. While they do provide immediate access to health care, their lack of knowledge and training leads to frequent misdiagnoses, overmedication and sometimes catastrophic outcomes.
Our Rural Health Kiosk model is an alternative service delivery model that offers primary care through an innovative ecosystem for just $1 per visit. We equip trained Health Assistants with our History-taking and Diagnostic Intelligence system, which guides them with initial history-taking in a manner that mirrors a doctor’s thought processes and transmits these results to a remotely located doctor. Armed with this information, the doctor can consult the patient, conduct further physical examination through the HA, and prescribe a treatment plan as if he or she were there in person.
Operating under a lean startup model, we have started two kiosks in West Bengal, seeing paying patients every day. With the maturation of the HDI, our model will grow, becoming a network comprising hundreds of kiosks to serve millions currently without access to qualified care.
-
The Team
Student Team: Kimber Ashman, Aaron Chang, Ian Graham, Nichaluk Leartprapun, Patience Osei, Mihika Reddy
Clinical Advisors: Barrett Yates, MSE, Sunny Chen, Tor Inge Garvik, MSc, Cherrie Evans, CNM, MSN, DrPH, Kusum Thapa, MD, FRCOG, MPH, Blami Dao, MD, Annie Clark, CNM, MPH, Harshad Sanghvi, MDAbstract
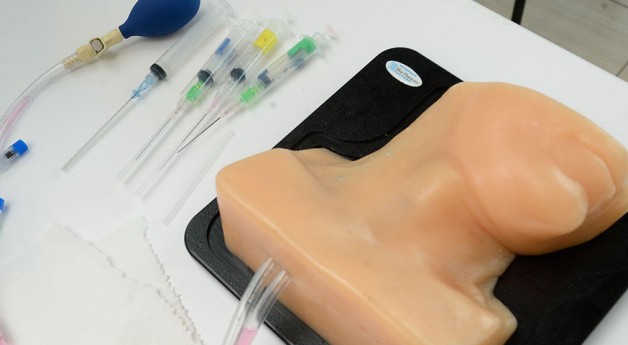
Many caesarean sections are preventable. During our August field immersion trips in Nepal and India, we witnessed high rates of caesarean sections and low rates of instrumental deliveries. In low- and middle-income countries, caesarean sections not only increase costs, but also maternal and fetal morbidities. From the discussions we have had with OBGYNs and midwives in India and Nepal, along with members in the public health sector, we have recognized that a key contributor to the high prevalence of caesarean sections is the lack of training in managing the progression of the second stage of labor and performing assisted deliveries. Many midwives and skilled birth attendants do not have the confidence to perform proper fetal head assessments during vaginal exams, which is critical to the decision-making process for appropriate referral for assisted delivery. Currently, there are no low-cost labor management training simulators for LMICs that adequately teach fetal head assessment.
Our team is creating a low-cost simulation tool that provides a method of safely practicing the vaginal examination skills that are essential to clinical decision making during the second stage of labor. Our device will help teach proper assessment of fetal head position, station, orientation, and moulding during a vaginal exam. We hope to enable midwives and SBAs to feel more confident in managing labor by allowing them to practice the skills that are vital to appropriate decision making and thereby prevent unnecessary caesarean sections.
U.S. Healthcare Innovations
-
The Team
Student Team: Wes Bernier, Trent Langston, Nichaluk Leartprapun, Qian Liu and Jackie Wanjala
Clinical Advisors: David Kass, MD, Peter Johnston, MD, Chao-Wei Hwang, MD PhD, Viachaslau Barodka, MD, Majd AlGhatrif, MD, Stuart Russell, MD, Soumyadipta Acharya, MD, PhD, Youseph Yazdi, MBA, PhD, Lawrence Aronhime, MBA, Matt Oberdier, PhD, Aditya Polsani, MS, Michael Parlato, MSE, Clifford Weiss, MD
Sponsor: Boston Scientific Team — Umang Anand, PhD, Andrew Bicek, PhD, Paul ChouinardAbstract
Heart failure affects the lives of 5.1 million Americans and cost the U.S. economy $39.2 Billion in 2010. An important aspect of managing HF is proper intravascular fluid optimization. The parameter that best quantifies fluid status is Left-Ventricular End-Diastolic Pressure. However, the current standards of care in obtaining fluid status assessment involve either a catheterization procedure that is accurate but inaccessible for regular use or inaccurate, nonspecific and subjective physical examinations. This inadequate assessment of a patient’s fluid status can lead to suboptimal medical dosing and, subsequently, increased incidences of rehospitalization. In recognition of this significant clinical need, CardiON is developing a safer, cheaper and more accurate way of monitoring LVEDP.
CardiON’s scientific insight comes from the observation of the interplay between various cardiovascular pressure, acoustic, and electrical waveforms. Using multiple noninvasive hemodynamic measurements, our system employs a patient-specific algorithm to derive LVEDP in real time. So far, the team has developed and validated its approach using retrospective human data and pig experiments. CardiON has been able to demonstrate high correlations of LVEDP values (R-square > 0.9, p < 0.001) with the new noninvasive approach as compared to the invasive gold standard. Furthermore, preliminary analysis demonstrated no clinically significant differences between the actual and predicted LVEDP values (Prediction S.E. <3mmHg). CardiON’s LVEDP values fall well within the precision and accuracy thresholds that clinicians desire. Johns Hopkins clinicians and CardiON’s Boston Scientific collaborators have expressed great excitement and anticipation in this new LVEDP monitor.
Moving forward, CardiON hopes to finalize the design of the prototype and continue validation of the patient specific algorithms in a way that informs possible future generalization. CardiON has obtained provisional patents on its designs and algorithms and hopes to begin pursuing regulatory approval (De Novo, Class II) in the near future. By enabling quantitative assessment of fluid status in all settings, CardiON hopes to revolutionize the way patients all over the world live with heart failure.
-
The Team
Student Team: Kimber Ashman, Brian Ma, Krairat Mairin, Mihika Reddy, Dhananjay Sethi
Clinical Advisors: Youseph Yazdi, PhD, Paul Segal, DO, Chris Jeffers, PhD, Steve Brooks, MD, Francisco Tejada, PhDAbstract
Proper hydration is a problem that is central to everyone’s health. Both overhydration and underhydration have detrimental medical consequences for various demographics. In the U.S. alone, more than half a million people will be hospitalized each year for preventable dehydration. Chronic patients, military personnel, and the elderly in particular need to be vigilant in monitoring their fluid intake. Fluid overload within our target patient population costs the health care system $7.6 billion per year. For military personnel, just a 4 percent decrease in hydration levels is enough to significantly degrade their physical and mental performance. However, there are currently no products on the market that continuously track hydration status.
MarsMedical is developing DrinkSync: a wearable, telemedicine-linked solution for monitoring hydration status. We output this result to the user, and our software will also track performance on your phone or computer, to help you build better habits over time. Users can take this information to see how their hydration levels change throughout the day. We can help you determine whether you’re under, over, or adequately hydrated and fuel your body accordingly. For chronic patients, the device will track the user’s actions and wirelessly communicate that information to central software, which will help doctors tailor treatments to the patient’s habits. With proper hydration, you can improve your cardiovascular function, exercise performance, cognitive function, and even mood.
-
The Team
Student Team: Aaron Chang, Allie Sibole, Madeleine Clegg, Patience Osei and Sriram Chadalavada
Clinical Advisors: Nevin M. Katz, MD, Viachaslau Barodka, MD, Derek M. Fine, MD, Dan E. Berkowitz, MBBCH, J. Trent Magruder, MD, Sharon Allan, RN, Steve Brooks, MD, Rengaswamy Srinivasan, PhD, Soumyadipta Acharya, MD, PhD, Youseph Yazdi, PhDAbstract
Acute kidney injury, defined as a sudden loss of renal function, occurs in 15 percent of the 600,000 cardiac procedures in the U.S. each year, leading to costly and morbid ICU stays. One of the most common causes is a drop in renal perfusion, leaving the kidney deprived of oxygen and unable to filter properly. As a result, the kidney decreases its urine production and increases its sodium retention. Kidney damage is potentially reversible through simple interventions, but the current gold standard detects the injury 48 hours too late. This has significant clinical and economic impact. Up to 2 percent of patients undergoing cardiac surgery leave the hospital requiring dialysis, and hospital-acquired acute kidney injury costs the U.S. health care system $10 billion each year.
The Renalert team is working with cardiac surgery, anesthesiology, and nephrology specialists at Johns Hopkins Hospital to facilitate the early detection of acute kidney injury. Our device, Renalert, provides real-time feedback about kidney function during cardiac surgery and in the intensive care unit. It uses continuous urinalysis to provide a patient-specific indication of kidney perfusion, and incorporates a unique algorithm of perioperative risk factors and hemodynamic parameters to assess the patient’s risk of acute kidney injury. Renalert detects declines in kidney function at the onset, alerting clinicians to make timely interventions that can prevent the associated complications of acute kidney injury.
https://www.youtube.com/watch?v=SDXH_3b5geo&feature=em-upload_owner
-
The Team
Student Team: David Blumenstyk, Ian Graham, Shruthi Rajan, Erin Reisfeld, Melody Tan
Clinical Advisors: Zoltan Mari, MD, Reza Shadmehr, PhD, Yousef Salimpour, PhD, Robert Atkinson, JD, Gad Alon, PT, PhD, Neil Rothman, PhD, Youseph Yazdi, PhD, MBA, Soumyadipta Acharya, MD, PhD, Paul Fearis, Lawrence Aronhime, MBA, Aditya Polsani, MS, Pratik Patel, MSE, William Anderson, MD, PhD, Kristen BowsherAbstract
Tremtex is working in conjunction with clinical and research partners at the Johns Hopkins School of Medicine to develop an intervention to help Parkinson’s disease patients manage their debilitating motor symptoms. Parkinson’s disease is an incurable, neurodegenerative disorder that affects more than 1 million people in the United States and 7 million people worldwide. The standard of care for Parkinson’s disease is medication; however, the effectiveness of medication wanes as the disease progresses. Another option for patients in advanced stages of the disease is deep brain stimulation, an expensive and invasive surgical procedure with strict eligibility criteria. This leaves a significant number of patients without an effective intervention.
Tremtex is addressing this gap by providing a low-risk solution for patients whose symptoms are undermanaged with existing interventions. The team has designed STIMband, a noninvasive electrical stimulation device, which delivers low and safe doses of transcranial direct current stimulation, to the motor cortices of the brain. The result is the reduction of motor symptoms, including tremors, and improved mobility. STIMband has been designed to ensure patient safety and usability, allowing patients to receive treatment within the comfort of their homes.
https://www.youtube.com/watch?v=vrpVYfQaevw&feature=em-upload_owner